Precision in Motion: A Breakthrough Study on Ballistic Tongue Evolution Across Species

The study by Zeng, Anderson & Deban (2025) is a masterclass in comparative biomechanics and evolutionary biology. Published in Current Biology, this article explores the convergent evolution of ballistic tongue mechanisms in salamanders and chameleons—two distantly related clades that have independently developed high-speed tongue projection systems. The authors dissect the structural and functional underpinnings of these systems, revealing a shared biomechanical principle: linear actuators powered by muscle compression of tapered skeletal rods.
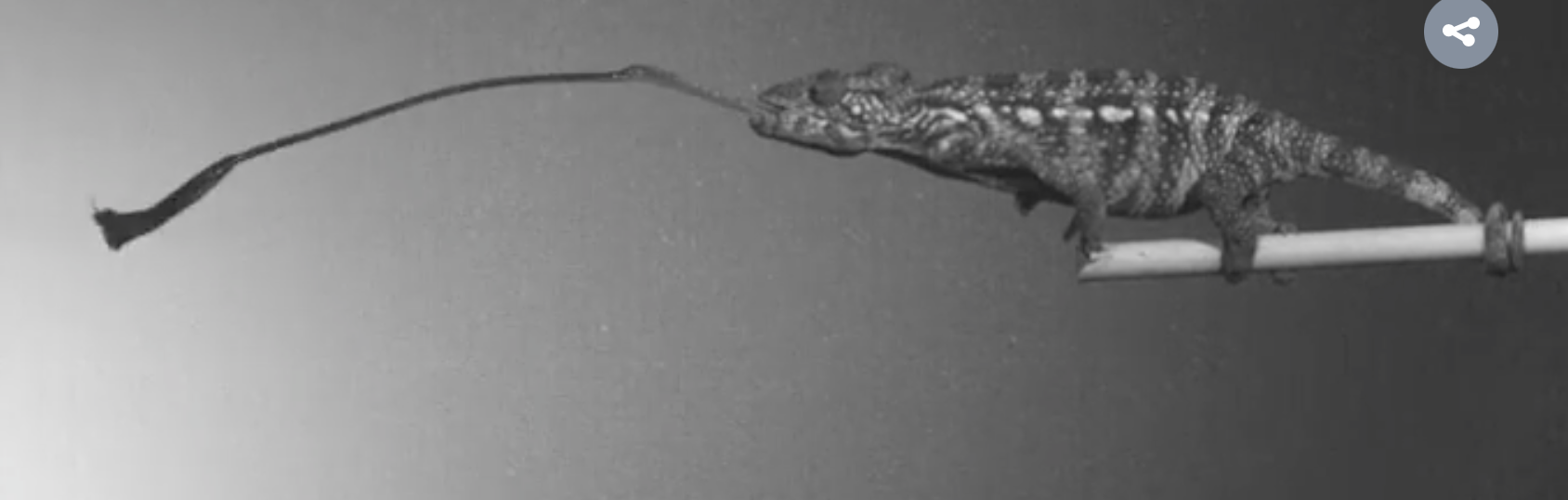
What makes this study exceptional is its synthesis of evolutionary theory with cutting-edge biomechanical analysis. Using high-speed videography, micro-CT imaging, and force transmission modeling, the authors demonstrate that both salamanders and chameleons have arrived at remarkably similar solutions for tongue projection, despite their divergent evolutionary paths. This convergence is not superficial—it extends to the internal architecture of the tongue apparatus, the dynamics of muscle contraction, and the physics of energy storage and release.
The article's central thesis is that slender muscle force transmission enables high-speed launches across a wide range of body sizes. This insight challenges previous assumptions that ballistic tongue performance is constrained by size or lineage. Instead, the authors show that both small and large species can achieve extraordinary projection speeds by optimizing the geometry and material properties of their tongue skeletons. This finding has broad implications for understanding functional constraints in vertebrate evolution.
One of the most compelling aspects of the study is its comparative framework. The authors do not treat chameleons and salamanders as isolated cases but as parallel experiments in nature's laboratory. By analyzing the mechanical properties of the entoglossal process in chameleons and the epibranchial rod in salamanders, they uncover a shared design principle: tapered rods that store elastic energy and release it explosively. This design allows for rapid tongue acceleration without relying on direct muscular speed, a key innovation in both groups.
The figures in the article are visually striking and scientifically rich. Figure 1 presents a side-by-side anatomical comparison of the tongue skeletons in representative chameleon and salamander species, highlighting the tapering and muscle attachment zones. Figure 2 shows high-speed footage frames of tongue projection, capturing the acceleration phase in exquisite detail. Figure 3 models the force transmission pathways and energy storage dynamics, offering a clear visualization of how muscle input translates into kinetic output. Figure 4 maps the evolutionary distribution of ballistic tongue systems across phylogenies, reinforcing the theme of convergence.
The authors also delve into the developmental and evolutionary origins of these systems. They propose that ballistic tongues arose through modular assembly of high-speed innovations, including muscle architecture, skeletal tapering, and elastic tissue integration. This modularity allowed different lineages to arrive at similar functional outcomes through distinct evolutionary routes—a concept that enriches our understanding of convergent evolution.
From a broader perspective, this study exemplifies the power of integrative biology. It combines morphology, physiology, physics, and evolutionary theory into a cohesive narrative that is both elegant and empirically grounded. The clarity of writing, the rigor of methodology, and the depth of insight make this article a landmark contribution to the field.
Zeng, Anderson & Deban (2025) have delivered a study that is as precise as the tongues it describes. By uncovering the shared biomechanical principles behind ballistic tongue projection, they have illuminated a fascinating example of evolutionary convergence. This work not only advances our understanding of vertebrate biomechanics but also sets a new standard for comparative functional morphology. It is a triumph of interdisciplinary science and a must-read for anyone interested in how evolution shapes performance.